

So far, we have no evidence that electrons have anything smaller rattling around inside them. Quarks are held together by the same strong force that holds the nucleus together (though the details of how it works in the two cases are a little different). The first is that if you hit protons and neutrons hard enough, you find that they in turn are made of even smaller pieces, called quarks. Two more points are probably worth covering while we're at it. Typically it takes about a million times as much energy to get stuff out of a nucleus as it does to strip an electron from an atom.Īlthough we've talked about breaking an atom apart in steps, you can, of course, hit a complete atom (electrons and nucleus) with something if you hit it hard enough, you'll get a load of bits and pieces. Thus, they can cruise straight into a nucleus, unimpeded by electromagnetic forces. Neutrons are particularly useful because they have no electric charge. Even so, the principle is the same: hit it either with photons (but now with those having much more energy than visible light photons) or any of the enormous zoo of particles discovered by high energy physicists. Because the strong force holding the protons and neutrons together is stronger than the electromagnetic one, knocking the nucleus apart into pieces demands more energy than removing the electrons.
Each successive electron tends to be harder to strip, as it sees itself as part of an object with an increasingly greater positive charge.Īfter all the electrons are gone, you're left with nothing but a nucleus. The first electron will come off fairly easily, leaving an object with a net positive charge (called an ion). It makes atoms move quickly and hit each other. Heat will do the trick too, but indirectly. Actually, light is made of little chunks called photons, so shining light on an atom is really just a special case of whacking it with other particles. Also, you can hit it with particles such as electrons or other atoms. You can shine light on the atom, or expose it to another form of electromagnetic radiation having an even shorter wavelength. The first thing you need to do is get rid of the electrons.

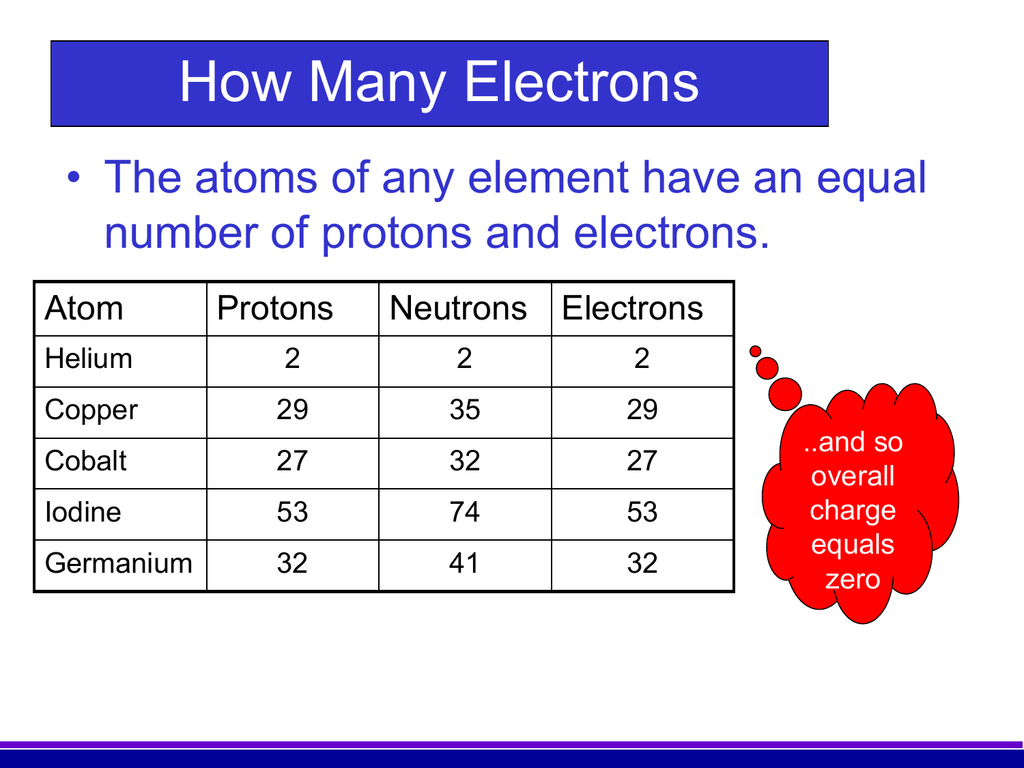
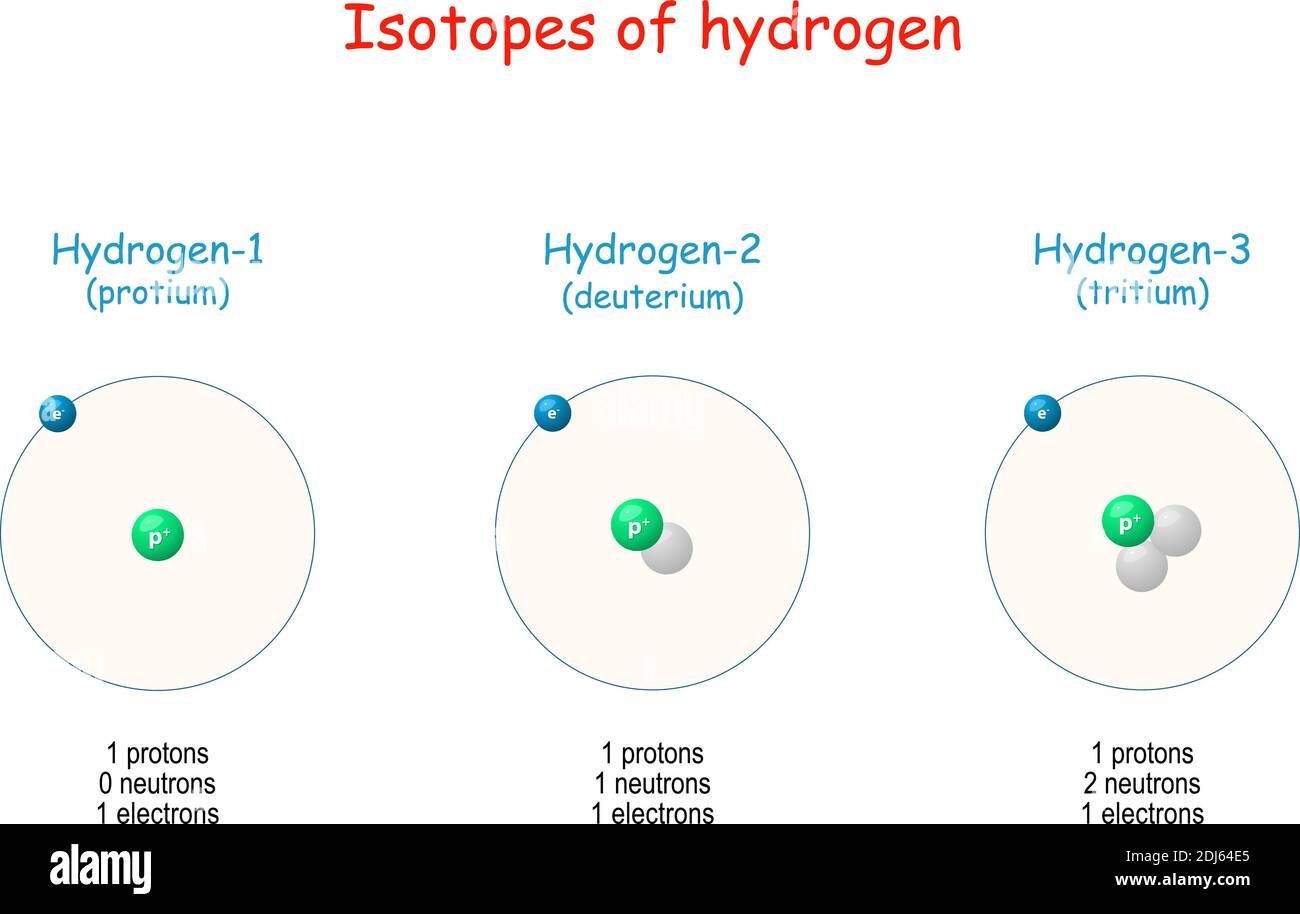
For most elements, there are several possibilities as to how many neutrons can fit into the nucleus, and each choice corresponds to a different isotope of that element. within the nucleus), a very strong force, more powerful than electromagnetism, takes over and attracts the protons and neutrons. Much like a proton in mass but without electric charge, the neutron is essential for holding the nucleus together. To avoid this separation, another particle comes into play in the nucleus: the neutron. The same electromagnetic force that draws opposite charged electrons and protons together tries to push the protons (which all have the same charge) away from each other. The number N tells you what element you have: for hydrogen N equals 1, for helium, 2, and so on. The force that holds the electrons and protons together is the electromagnetic force. Basically, it contains a nucleus, holding some number (call it N) of positively charged protons, which is surrounded by a cloud (N) of negatively charged electrons. Swain, professors at the Department of Physics of Northeastern University.įirst it's probably a good idea to review of what an atom is made of. This explanation is provided by the team of Stephen Reucroft and John D.


 0 kommentar(er)
0 kommentar(er)
